Territorial Availability: Available through Bertin Technologies only in France
- Synonyms
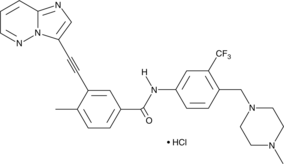
- 3-(2-imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-[4-[(4-methyl-1-piperazinyl)methyl]-3-(trifluoromethyl)phenyl]-benzamide, monohydrochloride
- Correlated keywords
- 943319-70-8 AP24534 HCL Iclusig BcrAbl BcrAblT315I BcrAbl T315I Bcr-Abl Q252H BcrAblQ252H BcrAblY253F Y253F BcrAblM351T M351T H396P BcrAblH396P IGF1R cSrc insulin Fibroblast growth factor receptor 1 Vascular endothelial growth factor platelet-derived growth factor receptor ? baf3 Ph+ALL
- Product Overview:
Ponatinib is an orally bioavailable Bcr-Abl tyrosine kinase inhibitor (IC50 = 0.37 nM).{25003} It inhibits the tyrosine kinase inhibitor-resistant mutant Bcr-AblT315I (IC50 = 2 nM), as well as Bcr-AblQ252H, Bcr-AblY253F, Bcr-AblM351T, and Bcr-AblH396P mutants (IC50s = 0.44, 0.3, 0.3, and 0.34 nM, respectively). It is selective for Bcr-Abl and these mutants over the insulin receptor, IGF-1R, Aurora A kinase, and Cdk2/cyclin E but does inhibit the receptor tyrosine kinases c-Src, VEGF receptor 2 (VEGFR2), FGFR1, and PDGFR? (IC50s = 5.4, 1.5, 2.2, and 1.1 nM, respectively). Ponatinib inhibits proliferation of Ba/F3 cells expressing native (IC50 = 0.5 nM) or mutant Bcr-Abl (IC50s = 0.5-36 nM) and induces apoptosis. It reduces tumor growth in a Ba/F3 Bcr-AblT315I mouse xenograft model when administered at doses ranging from 10 to 30 mg/kg. Formulations containing ponatinib have been used in the treatment of chronic-, accelerated-, or blast-phase chronic myeloid leukemia (CML), T315I-positive CML, or T315I-positive Philadelphia-chromosome positive acute lymphoblastic leukemia (Ph+ ALL).
Cayman Chemical’s mission is to help make research possible by supplying scientists worldwide with the basic research tools necessary for advancing human and animal health. Our utmost commitment to healthcare researchers is to offer the highest quality products with an affordable pricing policy.
Our scientists are experts in the synthesis, purification, and characterization of biochemicals ranging from small drug-like heterocycles to complex biolipids, fatty acids, and many others. We are also highly skilled in all aspects of assay and antibody development, protein expression, crystallization, and structure determination.
Over the past thirty years, Cayman developed a deep knowledge base in lipid biochemistry, including research involving the arachidonic acid cascade, inositol phosphates, and cannabinoids. This knowledge enabled the production of reagents of exceptional quality for cancer, oxidative injury, epigenetics, neuroscience, inflammation, metabolism, and many additional lines of research.
Our organic and analytical chemists specialize in the rapid development of manufacturing processes and analytical methods to carry out clinical and commercial GMP-API production. Pre-clinical drug discovery efforts are currently underway in the areas of bone restoration and repair, muscular dystrophy, oncology, and inflammation. A separate group of Ph.D.-level scientists are dedicated to offering Hit-to-Lead Discovery and Profiling Services for epigenetic targets. Our knowledgeable chemists can be contracted to perform complete sample analysis for analytes measured by the majority of our assays. We also offer a wide range of analytical services using LC-MS/MS, HPLC, GC, and many other techniques.
Accreditations
ISO/IEC 17025:2005
ISO Guide 34:2009
Cayman is a leader in the field of emerging drugs of abuse, providing high-purity Schedule I-V Controlled Substances to federally-licensed laboratories and qualified academic research institutions for forensic analyses. We are certified by ACLASS Accreditation Services with dual accreditation to ISO/IEC 17025:2005 and ISO Guide 34:2009.