Territorial Availability: Available through Bertin Technologies only in France
- Synonyms
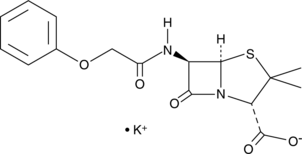
- (2S,5R,6R)-3,3-dimethyl-7-oxo-6-[(2-phenoxyacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, monopotassium salt
- Correlated keywords
- 87-08-1 8059-73-2 anti-biotic Clostridium Staphylococcus streptococcus Abbocillin VK V-K Acipen Anapenil Antibiocin Apo-Pen Apsin Arcacil Arcasin Asillin Beapen Beepen Beromycin 400 Betapen V Calciopen K Cilacil Cilicaine Cliacil Compocillin DQV-K Distakaps Distaquaine Dowpen DuraPenicillin Fenocin Fenocin Forte Fenospen Fenoxcillin Fenoxypen Icipen Isocillin Ispenoral Kavepenin L.P.V. Ledercillin Len Megacilina Megacillin Microcillin Micropen Milcopen Monocillin E Nadopen Newcillin Novo Novopen Ocillin Oracil Oracillin Orapen Orvek Ospen 250 KV Ospeneff P.V.O. PVK Pedipen Pen-V Pen-Vee K Powder Pen-Vee-K Pen-Vi-K Penadur Penagen Penapar Pencompren Penivoral Penoxilin Pentabs Pentacillin Pentranex Penvikal Penvisil Primcillin Qidpen Rafapen Robicillin Rocilin Rocillin Roscopenin SK Servipen Stabilin Sumapen Suspen Trepopen Uticillin V-Cillin V-Kal V-Pen Kalium Vamosyn Veetids Vepen Vepicombin phenoxymethylpenicillinate phenoxymethylpenicillinic
- Product Overview:
Penicillin V is a ?-lactam antibiotic that inhibits the growth of bacteria.{36398,36399,36400} In vitro, penicillin V inhibits the growth of clinical isolates of Streptococci (MICs = 0.004-0.008 mg/l) and C. difficile (MICs = 1 to >256 mg/l).{36398,36399} Penicillin V also inhibits growth of S. aureus in vitro (MIC = 0.016 mg/l) and in vivo in a thigh model of infection in mice.{36400} It inhibits the growth of S. pyogenes in mice following one or two dose therapy with curative dose (CD50) values of 0.207 and 0.031 mg, respectively.{36401} Penicillin V (100 mg/kg per day, p.o.) prevents experimental acute otitis media in rats when administered prior to infection by pneumococci.{36402} Formulations containing penicillin V have been used to treat bacterial infections of the skin, throat, ear, mouth, and respiratory tract.
Cayman Chemical’s mission is to help make research possible by supplying scientists worldwide with the basic research tools necessary for advancing human and animal health. Our utmost commitment to healthcare researchers is to offer the highest quality products with an affordable pricing policy.
Our scientists are experts in the synthesis, purification, and characterization of biochemicals ranging from small drug-like heterocycles to complex biolipids, fatty acids, and many others. We are also highly skilled in all aspects of assay and antibody development, protein expression, crystallization, and structure determination.
Over the past thirty years, Cayman developed a deep knowledge base in lipid biochemistry, including research involving the arachidonic acid cascade, inositol phosphates, and cannabinoids. This knowledge enabled the production of reagents of exceptional quality for cancer, oxidative injury, epigenetics, neuroscience, inflammation, metabolism, and many additional lines of research.
Our organic and analytical chemists specialize in the rapid development of manufacturing processes and analytical methods to carry out clinical and commercial GMP-API production. Pre-clinical drug discovery efforts are currently underway in the areas of bone restoration and repair, muscular dystrophy, oncology, and inflammation. A separate group of Ph.D.-level scientists are dedicated to offering Hit-to-Lead Discovery and Profiling Services for epigenetic targets. Our knowledgeable chemists can be contracted to perform complete sample analysis for analytes measured by the majority of our assays. We also offer a wide range of analytical services using LC-MS/MS, HPLC, GC, and many other techniques.
Accreditations
ISO/IEC 17025:2005
ISO Guide 34:2009
Cayman is a leader in the field of emerging drugs of abuse, providing high-purity Schedule I-V Controlled Substances to federally-licensed laboratories and qualified academic research institutions for forensic analyses. We are certified by ACLASS Accreditation Services with dual accreditation to ISO/IEC 17025:2005 and ISO Guide 34:2009.