Territorial Availability: Available through Bertin Technologies only in France
- Correlated keywords
- MC-4R melanocortins petides hormones proopiomelanocortins pituitary CNS central nervous systems skin placenta tissues MCR receptors G protein-coupled GPCRs cAMP Adenosine 3′,5′ cyclic mononucleotides messenger signal transduction pathways melanocortin-3 hypothalmus homeostasis fat lean mass hyperphagia MC4R melanocortin-4 adiposity MC3R-knockout mice mouse murine fatty acids oxidations blood pressure hypertension obesity glucose metabolisms insulin insensitivity agonists ligands sexual function antagonists fever suppression inflammatory mediators in vivo Surface transfection and expression protocols solid phase transient transfection technology originus patents USPT US6897067 US6902933 US7056741 US 6897067 6902933 7056741 DNA deoxyribonucleases adherent cells receptor-mediated endocytosis solution-phase co-transfection plasmids secretory alkalines phosphatases substrates SEAP proteins lipids signal transduction luminescence-based cell-based assays kits CBAs high-throughput screening a-MSH alpha-MSH ?-MSH reverse reporter
- Product Overview:
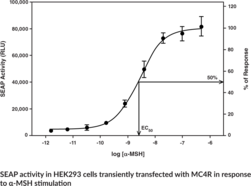
The melanocortin-4 receptor (MC4R) has important roles in weight regulation, sexual function, and inflammation. Mice deficient in MC4R have increased lipid deposition associated with elevated adiposity, while mutations in MC4R in humans are associated with early onset or severe obesity. This assay consists of a 96-well plate coated with a transfection complex containing DNA constructs for MC4R and a cAMP response element regulated Secreted Alkaline Phosphatase (SEAP) reporter (MC4R Reverse Transfection Strip Plate). Cells grown on the transfection complex will express MC4R at the cell surface. Binding of agonists to MC4R initiates a signal transduction cascade resulting in expression of SEAP which is secreted into the cell culture media. SEAP activity is measured following addition of a luminescence-based alkaline phosphatase substrate provided in the kit.
Cayman Chemical’s mission is to help make research possible by supplying scientists worldwide with the basic research tools necessary for advancing human and animal health. Our utmost commitment to healthcare researchers is to offer the highest quality products with an affordable pricing policy.
Our scientists are experts in the synthesis, purification, and characterization of biochemicals ranging from small drug-like heterocycles to complex biolipids, fatty acids, and many others. We are also highly skilled in all aspects of assay and antibody development, protein expression, crystallization, and structure determination.
Over the past thirty years, Cayman developed a deep knowledge base in lipid biochemistry, including research involving the arachidonic acid cascade, inositol phosphates, and cannabinoids. This knowledge enabled the production of reagents of exceptional quality for cancer, oxidative injury, epigenetics, neuroscience, inflammation, metabolism, and many additional lines of research.
Our organic and analytical chemists specialize in the rapid development of manufacturing processes and analytical methods to carry out clinical and commercial GMP-API production. Pre-clinical drug discovery efforts are currently underway in the areas of bone restoration and repair, muscular dystrophy, oncology, and inflammation. A separate group of Ph.D.-level scientists are dedicated to offering Hit-to-Lead Discovery and Profiling Services for epigenetic targets. Our knowledgeable chemists can be contracted to perform complete sample analysis for analytes measured by the majority of our assays. We also offer a wide range of analytical services using LC-MS/MS, HPLC, GC, and many other techniques.
Accreditations
ISO/IEC 17025:2005
ISO Guide 34:2009
Cayman is a leader in the field of emerging drugs of abuse, providing high-purity Schedule I-V Controlled Substances to federally-licensed laboratories and qualified academic research institutions for forensic analyses. We are certified by ACLASS Accreditation Services with dual accreditation to ISO/IEC 17025:2005 and ISO Guide 34:2009.