Territorial Availability: Available through Bertin Technologies only in France
- Correlated keywords
- Metallo proteinase endo peptidase GelatinaseB MANDP 2 Metallopeptidase9 Metalloprotease9 Metalloproteinase9 Q 29 R Q29 R 29R Glu 279 NF?B AP1 Metallopeptidase Metalloprotease MMP9 MMP 9
- Product Overview:
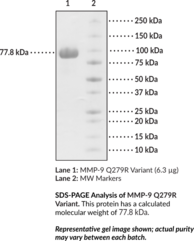
Matrix metalloproteinase-9 (MMP-9) is an endopeptidase and a member of the type IV collagenase subfamily of MMPs that has a major role in tissue remodeling.{61159} It is composed of an N-terminal prodomain containing a cysteine switch that interacts with the catalytic domain to regulate the proteolytic activity of MMP-9, a fibronectin-like domain that binds various extracellular matrix (ECM) proteins, and a C-terminal hemopexin-like domain that inhibits the proteolytic activity of MMP-9 by binding tissue inhibitor of metalloproteinases (TIMPs). The Q279R variant of MMP-9 possesses a glutamine-to-arginine substitution at Glu279, which is found in the substrate-binding region of the fibronectin-like domain.{61160} MMP-9 is synthesized and secreted as an inactive enzyme by a variety of cells, including neutrophils, macrophages, and fibroblasts, and is activated in the extracellular space by proteolytic cleavage of the prodomain by several proteases.{61161,61159,61162} MMP9 expression is regulated by the transcription factors NF-?B and AP-1, which can be induced by a variety of biological mediators, including reactive oxygen species (ROS) and inflammatory cytokines.{61159} MMP-9 participates in ECM remodeling, a process that is critical for development and wound healing, by degrading a variety of ECM proteins, including collagens and gelatins. Increased MMP-9 activity has been observed in a variety of pathological conditions, including cardiovascular diseases, arthritis, and cancer. The MMP-9 Q279R variant has been found in patients with polycythemia vera, essential thrombocytosis, or idiopathic myelofibrosis.{61160} Cayman’s MMP-9 Q279R Variant (human, recombinant) protein can be used for enzyme activity applications.
Cayman Chemical’s mission is to help make research possible by supplying scientists worldwide with the basic research tools necessary for advancing human and animal health. Our utmost commitment to healthcare researchers is to offer the highest quality products with an affordable pricing policy.
Our scientists are experts in the synthesis, purification, and characterization of biochemicals ranging from small drug-like heterocycles to complex biolipids, fatty acids, and many others. We are also highly skilled in all aspects of assay and antibody development, protein expression, crystallization, and structure determination.
Over the past thirty years, Cayman developed a deep knowledge base in lipid biochemistry, including research involving the arachidonic acid cascade, inositol phosphates, and cannabinoids. This knowledge enabled the production of reagents of exceptional quality for cancer, oxidative injury, epigenetics, neuroscience, inflammation, metabolism, and many additional lines of research.
Our organic and analytical chemists specialize in the rapid development of manufacturing processes and analytical methods to carry out clinical and commercial GMP-API production. Pre-clinical drug discovery efforts are currently underway in the areas of bone restoration and repair, muscular dystrophy, oncology, and inflammation. A separate group of Ph.D.-level scientists are dedicated to offering Hit-to-Lead Discovery and Profiling Services for epigenetic targets. Our knowledgeable chemists can be contracted to perform complete sample analysis for analytes measured by the majority of our assays. We also offer a wide range of analytical services using LC-MS/MS, HPLC, GC, and many other techniques.
Accreditations
ISO/IEC 17025:2005
ISO Guide 34:2009
Cayman is a leader in the field of emerging drugs of abuse, providing high-purity Schedule I-V Controlled Substances to federally-licensed laboratories and qualified academic research institutions for forensic analyses. We are certified by ACLASS Accreditation Services with dual accreditation to ISO/IEC 17025:2005 and ISO Guide 34:2009.