Territorial Availability: Available through Bertin Technologies only in France
- Synonyms
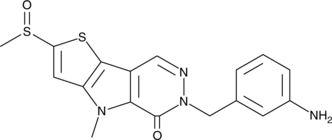
- 6-[(3-aminophenyl)methyl]-4,6-dihydro-4-methyl-2-(methylsulfinyl)-5H-thieno[2’,3’:4,5]pyrrolo[2,3-d]pyridazin-5-one
- Correlated keywords
- aerobic glycolysis pyruvate kinases PKM2 antitumors antiproliferation activators anticancers anti-tumors anti tumors cancers anti-cancers anti-proliferation proliferation ML265 MSLs 265 tumor-specific PKMs PKM-2 2 two fructose-1,6-bisphosphate 1,6-FBP FBPs binding binds binders dimer-dimer dimers interfaces tetramerization isoforms reduces reduction H1299 H-1299 Hs 1299 cells xenografts lungs carcinoma CIDs CID-44246499 CID44246499 NCGC-00186528 NCGCs 00186528 NCGC00186528 186528 TEPPs TEPP46 TEPP-46 46 metabolism catalyzes final steps formations ATP phosphoenolpyruvate phosphosenol ADP expressions M2 M 2 isozymes PK metabolic reprogramming glucose allosterically allosteric regulated regulates upstream up-stream up streams glycolytic intermediates fructose 1 6 bisphosphate one six feed forward mechanisms phosphorylated states peptides motifs tyrosines inhibits inhibitors inhibitions activity releases sites ML-265 specific subunits inducing induces active forms enzymes folds selectivity PKM1 PKR PKL PRM-1 M1 1 sizes weights occurrences mice models humans non-small nonsmall non small
- Product Overview:
Pyruvate kinase catalyzes the final step in glycolysis, the formation of pyruvate and ATP from phosphoenolpyruvate and ADP. The expression of the M2 isozyme of pyruvate kinase (PKM2) plays an important role in the metabolic reprogramming of tumor cells, which require high amounts of glucose for proliferation. PKM2 is allosterically regulated by the upstream glycolytic intermediate, fructose-1,6-bisphosphate (FBP), which controls glycolysis in a feed forward mechanism.{21504} Whereas cancer cells exist in highly phosphorylated states, the binding of certain peptide motifs with phosphorylated tyrosines can inhibit PKM2 activity by causing the release of FBP from the allosteric site.{21504} ML-265 activates tumor-specific PKM2 (EC50 = 92 nM) by binding to the dimer-dimer interface between two subunits of PKM2 and inducing tetramerization, which is the most active form of the enzyme.{24497} It demonstrates >100-fold selectivity for PKM2 over the related PKM1, PKR, and PKL isoforms.{24497} At 50 mg/kg, ML-265 has been shown to reduce tumor size, weight, and occurrence in mice bearing H1299 cell xenografts in a model of human non-small cell lung carcinoma.{24497}
Cayman Chemical’s mission is to help make research possible by supplying scientists worldwide with the basic research tools necessary for advancing human and animal health. Our utmost commitment to healthcare researchers is to offer the highest quality products with an affordable pricing policy.
Our scientists are experts in the synthesis, purification, and characterization of biochemicals ranging from small drug-like heterocycles to complex biolipids, fatty acids, and many others. We are also highly skilled in all aspects of assay and antibody development, protein expression, crystallization, and structure determination.
Over the past thirty years, Cayman developed a deep knowledge base in lipid biochemistry, including research involving the arachidonic acid cascade, inositol phosphates, and cannabinoids. This knowledge enabled the production of reagents of exceptional quality for cancer, oxidative injury, epigenetics, neuroscience, inflammation, metabolism, and many additional lines of research.
Our organic and analytical chemists specialize in the rapid development of manufacturing processes and analytical methods to carry out clinical and commercial GMP-API production. Pre-clinical drug discovery efforts are currently underway in the areas of bone restoration and repair, muscular dystrophy, oncology, and inflammation. A separate group of Ph.D.-level scientists are dedicated to offering Hit-to-Lead Discovery and Profiling Services for epigenetic targets. Our knowledgeable chemists can be contracted to perform complete sample analysis for analytes measured by the majority of our assays. We also offer a wide range of analytical services using LC-MS/MS, HPLC, GC, and many other techniques.
Accreditations
ISO/IEC 17025:2005
ISO Guide 34:2009
Cayman is a leader in the field of emerging drugs of abuse, providing high-purity Schedule I-V Controlled Substances to federally-licensed laboratories and qualified academic research institutions for forensic analyses. We are certified by ACLASS Accreditation Services with dual accreditation to ISO/IEC 17025:2005 and ISO Guide 34:2009.