Territorial Availability: Available through Bertin Technologies only in France
- Synonyms
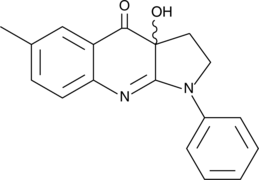
- 1,2,3,3a-tetrahydro-3a-hydroxy-6-methyl-1-phenyl-4H-pyrrolo[2,3-b]quinolin-4-one
- Correlated keywords
- inhibitors muscles myosin ATPase smooth bleb apoptosis cells migration chemotaxis cytokinesis division growth UV lights
- Product Overview:
(±)-Blebbistatin is a racemic mixture of (–)-blebbistatin (Item No. 13013) and (+)-blebbistatin (Item No. 13165), the active and inactive forms, respectively. (–)-Blebbistatin is a selective cell-permeable inhibitor of non-muscle myosin II ATPases.{16452,17037} (–)-Blebbistatin rapidly and reversibly inhibits Mg-ATPase activity and in vitro motility of non-muscle myosin IIA and IIB for several species (IC50s = 0.5-5 µM), while poorly inhibiting smooth muscle myosin (IC50 = 8 µM).{17036} Through these effects, (–)-blebbistatin blocks apoptosis-related bleb formation, directed cell migration, and cytokinesis in vertebrate cells. However, prolonged exposure to blue light (450-490 nm) results in degradation of blebbistatin to an inactive product via cytotoxic intermediates, which may be problematic for its use in fluorescent live cell imaging applications.{17038,24037} (S)-nitro-Blebbistatin (Item No. 13891) and (R)-nitro-blebbistatin (Item No. 9001935) are more stable forms of (–)-blebbistatin and (+)-blebbistatin, respectively. The addition of a nitro group stabilizes the molecules to circumvent their degradation by prolonged blue light exposure.{16725} (S)-nitro-Blebbistatin and (R)-nitro-blebbistatin have the same stereochemistry of the active and inactive enantiomers, respectively.
Cayman Chemical’s mission is to help make research possible by supplying scientists worldwide with the basic research tools necessary for advancing human and animal health. Our utmost commitment to healthcare researchers is to offer the highest quality products with an affordable pricing policy.
Our scientists are experts in the synthesis, purification, and characterization of biochemicals ranging from small drug-like heterocycles to complex biolipids, fatty acids, and many others. We are also highly skilled in all aspects of assay and antibody development, protein expression, crystallization, and structure determination.
Over the past thirty years, Cayman developed a deep knowledge base in lipid biochemistry, including research involving the arachidonic acid cascade, inositol phosphates, and cannabinoids. This knowledge enabled the production of reagents of exceptional quality for cancer, oxidative injury, epigenetics, neuroscience, inflammation, metabolism, and many additional lines of research.
Our organic and analytical chemists specialize in the rapid development of manufacturing processes and analytical methods to carry out clinical and commercial GMP-API production. Pre-clinical drug discovery efforts are currently underway in the areas of bone restoration and repair, muscular dystrophy, oncology, and inflammation. A separate group of Ph.D.-level scientists are dedicated to offering Hit-to-Lead Discovery and Profiling Services for epigenetic targets. Our knowledgeable chemists can be contracted to perform complete sample analysis for analytes measured by the majority of our assays. We also offer a wide range of analytical services using LC-MS/MS, HPLC, GC, and many other techniques.
Accreditations
ISO/IEC 17025:2005
ISO Guide 34:2009
Cayman is a leader in the field of emerging drugs of abuse, providing high-purity Schedule I-V Controlled Substances to federally-licensed laboratories and qualified academic research institutions for forensic analyses. We are certified by ACLASS Accreditation Services with dual accreditation to ISO/IEC 17025:2005 and ISO Guide 34:2009.