Territorial Availability: Available through Bertin Technologies only in France
- Synonyms
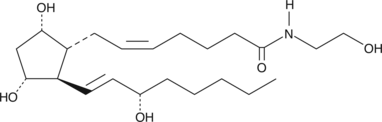
- N-(2-hydroxyethyl)-9?,11?,15S-trihydroxy-prosta-5Z,13E-dien-1-amide
- Correlated keywords
- PGF2alpha anandamide COX-2 cox2 cyclooxygenases pghs2 pghs-2 synthase-2 neuroscience MS quantitative standard mass Max spec spectrometry lipidomics LC-MS LCMS
- Product Overview:
Prostaglandin F2? ethanolamide (PGF2?-EA) is produced by cyclooxygenase 2 (COX-2) metabolism of the endogenous cannabinoid, arachidonoyl ethanolamide (AEA; Item No. 90050), found in brain, liver, and other mammalian tissues.{2415} AEA can be metabolized directly by the sequential action of COX-2 and specific PG synthases to produce ethanolamide congeners of the classical PGs.{5089,11045} PGF2?-EA has also been reported to be biosynthesized by this mechanism when AEA was infused into the lung and liver of living mice. PGF2?-EA is a potent dilator (EC50 = 58 nM) of the cat iris sphincter, which is a model system for testing potential intraocular hypotensive agents.{8671} PGF2?-EA MaxSpec® standard is a quantitative grade standard of PGF2?-EA (Item No. 16013) that has been prepared specifically for mass spectrometry or any application where quantitative reproducibility is required. The solution has been prepared gravimetrically and is supplied in a deactivated glass ampule sealed under argon. The concentration was verified by comparison to an independently prepared calibration standard. This PGF2?-EA MaxSpec® standard is guaranteed to meet identity, purity, stability, and concentration specifications and is provided with a batch-specific certificate of analysis. Ongoing stability testing is performed to ensure the concentration remains accurate throughout the shelf life of the product. Note: The amount of solution added to the vial is in excess of the listed amount. Therefore, it is necessary to accurately measure volumes for preparation of calibration standards. Follow recommended storage and handling conditions to maintain product quality.
Cayman Chemical’s mission is to help make research possible by supplying scientists worldwide with the basic research tools necessary for advancing human and animal health. Our utmost commitment to healthcare researchers is to offer the highest quality products with an affordable pricing policy.
Our scientists are experts in the synthesis, purification, and characterization of biochemicals ranging from small drug-like heterocycles to complex biolipids, fatty acids, and many others. We are also highly skilled in all aspects of assay and antibody development, protein expression, crystallization, and structure determination.
Over the past thirty years, Cayman developed a deep knowledge base in lipid biochemistry, including research involving the arachidonic acid cascade, inositol phosphates, and cannabinoids. This knowledge enabled the production of reagents of exceptional quality for cancer, oxidative injury, epigenetics, neuroscience, inflammation, metabolism, and many additional lines of research.
Our organic and analytical chemists specialize in the rapid development of manufacturing processes and analytical methods to carry out clinical and commercial GMP-API production. Pre-clinical drug discovery efforts are currently underway in the areas of bone restoration and repair, muscular dystrophy, oncology, and inflammation. A separate group of Ph.D.-level scientists are dedicated to offering Hit-to-Lead Discovery and Profiling Services for epigenetic targets. Our knowledgeable chemists can be contracted to perform complete sample analysis for analytes measured by the majority of our assays. We also offer a wide range of analytical services using LC-MS/MS, HPLC, GC, and many other techniques.
Accreditations
ISO/IEC 17025:2005
ISO Guide 34:2009
Cayman is a leader in the field of emerging drugs of abuse, providing high-purity Schedule I-V Controlled Substances to federally-licensed laboratories and qualified academic research institutions for forensic analyses. We are certified by ACLASS Accreditation Services with dual accreditation to ISO/IEC 17025:2005 and ISO Guide 34:2009.