Territorial Availability: Available through Bertin Technologies only in France
- Correlated keywords
- hypercretins 1 orexins 2 systems regulates regulator sleep wake cycles feeding cardiovascular autonomic ANS hypothalamic neuropeptides A B gut hormones secretions hypothalamus midbrains brainstems G protein-coupled receptors GPCRs orexin-1 OX1R orexin-2 OX2R orexin-A orexin-B sleep disorders narcolepsy humans animals rodents energy homeostasis pain stress antagonists drugs addictions withdrawals therapeutics obesity Surface transfections and expressions protocols solid phase transient transfection technology originus patents USPT US6897067 US6902933 US7056741 US 6897067 6902933 7056741 DNA deoxyribonucleases adherent cells receptor-mediated luminescent secretory alkaline phosphatases SEAP signals transductions cascades culture medium luminescence-based high throughput HTS screening screens agonists reverse reporter assay
- Product Overview:
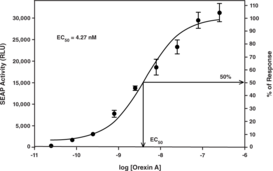
The Orexin 2 Receptor (OX2R) may be an important therapeutic target for treatment of sleep disorders, obesity, emotional stress, and addiction. Cayman’s OX2R Reporter Assay Kit consists of a 96-well plate coated with both OX2R and Secreted Alkaline Phosphatase (SEAP) reporter constructs (OX2R Reverse Transfection Strip Plate). Cells grown on the transfection complex will express OX2R at the cell surface and the recombinant G-protein inside the cell. Binding of agonists to OX2R initiates a signal transduction cascade resulting in expression of SEAP which is secreted into the cell culture medium. Aliquots of culture medium are removed at time intervals beginning at about 16 hours and SEAP activity is measured following addition of a luminescence-based alkaline phosphatases substrate provided in the kit. The kit is easy to use and can be easily adapted to high throughput screening for therapeutic compounds regulating activation of OX2R. A known OX2R agonist, Orexin A, is included in the kit for use as a positive control. The kit provides sufficient reagent to measure SEAP activity at three time points, using the black plates provided.
Cayman Chemical’s mission is to help make research possible by supplying scientists worldwide with the basic research tools necessary for advancing human and animal health. Our utmost commitment to healthcare researchers is to offer the highest quality products with an affordable pricing policy.
Our scientists are experts in the synthesis, purification, and characterization of biochemicals ranging from small drug-like heterocycles to complex biolipids, fatty acids, and many others. We are also highly skilled in all aspects of assay and antibody development, protein expression, crystallization, and structure determination.
Over the past thirty years, Cayman developed a deep knowledge base in lipid biochemistry, including research involving the arachidonic acid cascade, inositol phosphates, and cannabinoids. This knowledge enabled the production of reagents of exceptional quality for cancer, oxidative injury, epigenetics, neuroscience, inflammation, metabolism, and many additional lines of research.
Our organic and analytical chemists specialize in the rapid development of manufacturing processes and analytical methods to carry out clinical and commercial GMP-API production. Pre-clinical drug discovery efforts are currently underway in the areas of bone restoration and repair, muscular dystrophy, oncology, and inflammation. A separate group of Ph.D.-level scientists are dedicated to offering Hit-to-Lead Discovery and Profiling Services for epigenetic targets. Our knowledgeable chemists can be contracted to perform complete sample analysis for analytes measured by the majority of our assays. We also offer a wide range of analytical services using LC-MS/MS, HPLC, GC, and many other techniques.
Accreditations
ISO/IEC 17025:2005
ISO Guide 34:2009
Cayman is a leader in the field of emerging drugs of abuse, providing high-purity Schedule I-V Controlled Substances to federally-licensed laboratories and qualified academic research institutions for forensic analyses. We are certified by ACLASS Accreditation Services with dual accreditation to ISO/IEC 17025:2005 and ISO Guide 34:2009.