Primary Eicosanoid HPLC Mixture
Primary Eicosanoid HPLC Mixture
This mixture contains the primary COX products produced by most mammalian tissues. Contents: Prostaglandin D2, Prostaglandin E2, 6-keto Prostaglandin F1?,...
From €433.00 €368.05
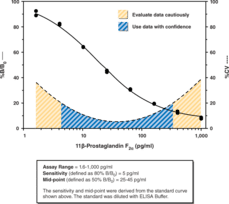
Prostaglandin D2 (PGD2) is the major eicosanoid released from stimulated mast cells. PGD2 is an unstable compound which is rapidly metabolized and eliminated from the circulation. This makes quantitation of PGD2 an unreliable indicator of its in vivo production. 11?-PGF2? is the primary plasma metabolite of PGD2 in vivo, the levels of which can increase from 6 pg/ml in a normal healthy volunteer to 490 ng/ml in patients with systemic mastocytosis.{493} The normal human urinary excretion of 11?-PGF2? is about 11 ng/mmol creatinine (~400 ng/24 hr), which is increased nearly 3-fold upon allergen-induced bronchoconstriction in asthmatics.{7577,3519} Unlike most prostaglandin metabolites, 11?-PGF2? retains potent biological activity. 11-?-PGF2? is equipotent to PGF2? in inducing human bronchial smooth muscle contractions and inhibition of adipose differentiation.{1756,2053} 11?-PGF2? was also shown to inhibit ADP or thrombin-induced human platelet aggregation at concentrations of 0.14 to 2.8 µM. {494} The Cayman 11?-PGF2? Assay is a competitive assay that provides accurate measurements of 11?-PGF2? within the range of 1.6-1,000 pg/ml. Inter and intra-assay CV’s of less than 15% can be achieved at most concentrations. This assay allows sensitive detection of 11?-PGF2? in the most common sample matrix, which is urine. For plasma and other complex sample matrices, we recommend purification of the sample prior to analysis; a purification protocol is included in the kit booklet. Measurements of 11?-PGF2? in urine using our ELISA gives values in the same range, but slightly higher than those obtained by GC-MS. This appears to be due to measurement of 11?-PGF2? plus the 2,3-dinor metabolite of 11?-PGF2?.{7577}
Territorial Availability: Available through Bertin Technologies only in France
| Size | 480 solid wells, 480 strip wells, 96 solid wells, 96 strip wells |
|---|---|
| Shipping | dry ice |
| Molecular weight | |
| Custom code | 3822.19 |
| UNSPSC code | 41116104 |
Cayman Chemical’s mission is to help make research possible by supplying scientists worldwide with the basic research tools necessary for advancing human and animal health. Our utmost commitment to healthcare researchers is to offer the highest quality products with an affordable pricing policy.
Our scientists are experts in the synthesis, purification, and characterization of biochemicals ranging from small drug-like heterocycles to complex biolipids, fatty acids, and many others. We are also highly skilled in all aspects of assay and antibody development, protein expression, crystallization, and structure determination.
Over the past thirty years, Cayman developed a deep knowledge base in lipid biochemistry, including research involving the arachidonic acid cascade, inositol phosphates, and cannabinoids. This knowledge enabled the production of reagents of exceptional quality for cancer, oxidative injury, epigenetics, neuroscience, inflammation, metabolism, and many additional lines of research.
Our organic and analytical chemists specialize in the rapid development of manufacturing processes and analytical methods to carry out clinical and commercial GMP-API production. Pre-clinical drug discovery efforts are currently underway in the areas of bone restoration and repair, muscular dystrophy, oncology, and inflammation. A separate group of Ph.D.-level scientists are dedicated to offering Hit-to-Lead Discovery and Profiling Services for epigenetic targets. Our knowledgeable chemists can be contracted to perform complete sample analysis for analytes measured by the majority of our assays. We also offer a wide range of analytical services using LC-MS/MS, HPLC, GC, and many other techniques.
Accreditations
ISO/IEC 17025:2005
ISO Guide 34:2009
Cayman is a leader in the field of emerging drugs of abuse, providing high-purity Schedule I-V Controlled Substances to federally-licensed laboratories and qualified academic research institutions for forensic analyses. We are certified by ACLASS Accreditation Services with dual accreditation to ISO/IEC 17025:2005 and ISO Guide 34:2009.
This mixture contains the primary COX products produced by most mammalian tissues. Contents: Prostaglandin D2, Prostaglandin E2, 6-keto Prostaglandin F1?,...
This mixture contains the primary metabolites of prostaglandins (PGs) D2, E2, and F2?. Contents: 13,14-dihydro-15-keto PGD2, 13,14-dihydro-15-keto PGE2, 11?-PGF2?, 13,14-dihydro-15-keto...
GST-tag Polyclonal Antibody (FITC) is a probe for the immunochemical detection of GST tags on recombinant proteins. Recombinant proteins are...
Amyloid-? (1-8, A2V) is a truncated form of amyloid-? (A?) that contains a valine to alanine substitution at position 2...
Inositol hexakisphosphate kinase 2 (IP6K2) is a cytoplasmic kinase that catalyzes the conversion of IP6 to diphosphoinositol pentakisphosphate in the...
Histone H2A is a core histone that forms a dimer with histone H2B.{46631} Two histone H2A/H2B dimers form an octameric...
Prostaglandin I synthase (PGIS) catalyzes the isomerization of PGH2 to PGI2. PGI2 (prostacyclin) is a potent vasodilator and inhibitor of...
This mixture contains the characteristic metabolites of both PGI2 and TXA2. Contents: Thromboxane B2, 11-dehydro Thromboxane B2, 6-keto Prostaglandin F1?,...
The cyclopentenone prostaglandin HPLC mixture contains all of the major UV-absorbing cyclopentenone prostaglandins and their precursors supplied in methyl acetate....
Protein phosphorylation is an important post-translational modification that serves many key functions to regulate a protein’s activity, localization, and protein-protein...
This mixture contains primary prostaglandins produced from arachidonic acid and dihomo-?-linolenic acid. Contents: Prostaglandin E1, Prostaglandin E2, Prostaglandin F1?, 6-keto...
Proprotein convertase subtilisin kexin 9 (PCSK9) is a member of the subtilisin serine protease family with an important role in...
To be used in conjunction with Cayman’s NAPE-PLD polyclonal antibody (aa 6-20) (Item No. 10306) to block protein-antibody complex formation...
The Cayman COX Inhibitor Pack contains a combination of frequently used cyclooxygenase (COX) inhibitors. Each kit contains aspirin, the archetype...
Programmed cell death protein 4 (PDCD4) levels are elevated during apoptosis and absent in many cancer samples.{16340,16167} Loss of PDCD4...
Platelet-activating factor (PAF) is an important lipid mediator involved in inflammation. PAF-acetylhydrolase (PAF-AH) is an extracellular phospholipase A2 which hydrolyzes...
To be used in conjunction with Cayman’s FABP3 polyclonal antibody (Catalog No. 10233) to block protein-antibody complex formation during immunochemical...
Sirtuin 5 (SIRT5) is an enzyme that catalyzes the NAD-dependent removal of malonyl, succinyl, and glutaryl groups from target proteins.{55107,55108}...
Headquarters:
Parc d’activités du Pas du Lac
10 bis avenue Ampère
78180 Montigny le Bretonneux
France
Copyright © 2024 BERTIN BIOREAGENT. All rights reserved | Terms & conditions