Territorial Availability: Available through Bertin Technologies only in France
- Synonyms
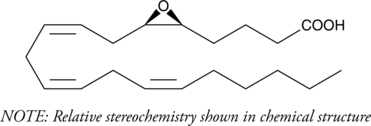
- (±)5,6-epoxy-8Z,11Z,14Z-eicosatrienoic acid
- Correlated keywords
- epoxides epoxygenases cytochrome P450 (+/-)5(6)-EpETrEs (+/-)5,6-EpETrEs 5,6-EETs fatty acids 132072-?49-?2 184488-43-5
- Product Overview:
5(6)-EET is a fully racemic version of the enantiomeric forms biosynthesized from arachidonic acid (Item No. 90010) by cytochrome P450 enzymes.{463,729} In solution, 5(6)-EET degrades into 5,6-DiHET and 5(6)-?-lactone, which can be converted to 5(6)-DiHET and quantified by GC-MS.{39654} In neuroendocrine cells, such as the anterior pituitary and pancreatic islets, 5(6)-EET has been implicated in the mobilization of calcium and hormone secretion.{462,460} 5(6)-EET is an inhibitor of T-type voltage-gated calcium channels (Cav3) that inhibits isoforms Cav3.1, Cav3.2 (IC50 = 0.54 µM), and Cav3.3 and decreases nifedipine-resistant phenylephrine-induced vasoconstriction in isolated mouse mesenteric arteries via Cav3.2 blockade when used at a concentration of 3 µM.{42110} In addition, it is a substrate of COX-1 and COX-2, as measured by oxygen consumption and product formation assays when used at a concentration of 50 µM.{34481} (±)5(6)-EET is provided as a mixture of the free acid and lactone.
Cayman Chemical’s mission is to help make research possible by supplying scientists worldwide with the basic research tools necessary for advancing human and animal health. Our utmost commitment to healthcare researchers is to offer the highest quality products with an affordable pricing policy.
Our scientists are experts in the synthesis, purification, and characterization of biochemicals ranging from small drug-like heterocycles to complex biolipids, fatty acids, and many others. We are also highly skilled in all aspects of assay and antibody development, protein expression, crystallization, and structure determination.
Over the past thirty years, Cayman developed a deep knowledge base in lipid biochemistry, including research involving the arachidonic acid cascade, inositol phosphates, and cannabinoids. This knowledge enabled the production of reagents of exceptional quality for cancer, oxidative injury, epigenetics, neuroscience, inflammation, metabolism, and many additional lines of research.
Our organic and analytical chemists specialize in the rapid development of manufacturing processes and analytical methods to carry out clinical and commercial GMP-API production. Pre-clinical drug discovery efforts are currently underway in the areas of bone restoration and repair, muscular dystrophy, oncology, and inflammation. A separate group of Ph.D.-level scientists are dedicated to offering Hit-to-Lead Discovery and Profiling Services for epigenetic targets. Our knowledgeable chemists can be contracted to perform complete sample analysis for analytes measured by the majority of our assays. We also offer a wide range of analytical services using LC-MS/MS, HPLC, GC, and many other techniques.
Accreditations
ISO/IEC 17025:2005
ISO Guide 34:2009
Cayman is a leader in the field of emerging drugs of abuse, providing high-purity Schedule I-V Controlled Substances to federally-licensed laboratories and qualified academic research institutions for forensic analyses. We are certified by ACLASS Accreditation Services with dual accreditation to ISO/IEC 17025:2005 and ISO Guide 34:2009.